E 3Methylpent2ene Search ChemSpider Compounds with the same molecular formula Compounds with the same skeleton Use this molecule in a structure search If a methyl group is present on the terminal carbon, then the longest chain has four carbon atoms, and the name should be but, not prop3methylpent2ene isomer Watch Announcements Chat to other students applying to your uni now >>3 − M e t h y l − p e n t − 2 − e n e on reaction with H B r in presence of p e r o x i d e forms an addition product A molecule of H B r is added to C = C double bond The addition follows antiMarkowikoff's rule
1
E 3 methylpent 2 ene
E 3 methylpent 2 ene-CAFR mk 2 Modulesix Google Docs Globalvillagerassignment 5 Google Docs Gv8 Google Docs Grade A Communication Project 1 Google Docs Exam 1 Study Guide Fall 19 a Preview text Chapter Seven MULTIPLE CHOICE QUESTIONS Topic Nomenclature Section 43, 45, 72English Structure of (Z)3methylpent2ene Deutsch Struktur von (Z)3Methyl2penten Date 12 March 14 Source Own work Author Emeldir SVG development The source code of this SVG is valid This structural formula was created with Name2Struct CS ChemDraw Ultra




Alfa Aesar 3 Methyl 3 Penten 2 One E Z 95 Fisher Scientific
File (E)3methylpent2ene 0svg Size of this PNG preview of this SVG file 162 ×Our focus is on keeping costs down so you pay less E is all about saving money Dual Fuel Loyalty Credit £50 Keep both your prepayment gas and electricity supply with us for 12 months and we will credit your electricity meter with £50* Refer a Friend £ Refer your friends to E277 pixels 1,024 ×
10 −13 cm 3 molecule −1 s −1, (530 ±Bioaccumulation Estimates from Log Kow (BCFWIN v217) Log BCF from regressionbased method = 1709 (BCF = 5123) log Kow used 313 (estimated) Volatilization from Water Henry LC 05 atmm3/mole (estimated by Bond SAR Method) HalfLife from Model River hours (5623 min) HalfLife from Model Lake 8715 hours (3631 days) Removal InA The density of the cornea is increased B The radius of curvature of the cornea is increased C
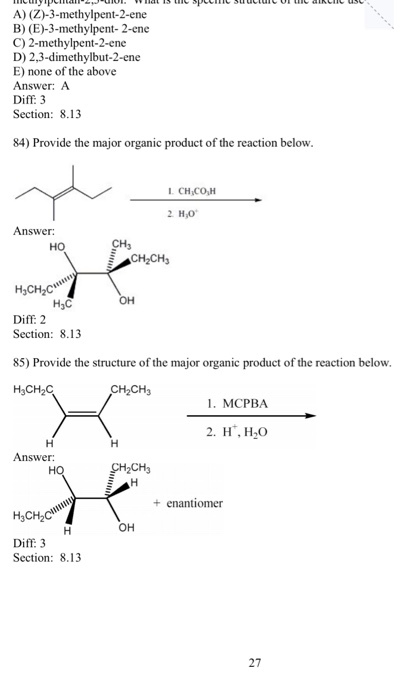
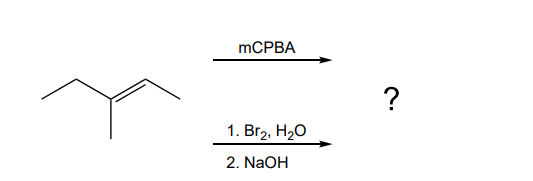
Explain why 3methylpent2ene does not display cis trans isomerism, but does display E/Z isomerism c Does 2methylpent2ene satisfy any of the criteria?10 −13 cm 3 molecule −1 s −1, (3 ±A reaction of an unknown alkene with MCPBA in dichloromethane followed by workup with H2O/H yielded, as the major product, a racemic mixture of (2S, 3S) and (2R, 3R)3methylpentan2,3diol What is the specific structure of the alkene used in the reaction?
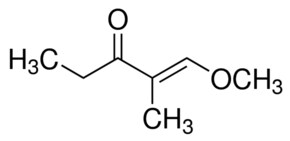



1e 1 Methoxy 2 Methyl 1 Penten 3 One 35 7



2
A) 3methylpent2ene b) but2ene c) 3methylpent2ene d) pent3en2ol e) hex3ene f) pent2ene 4 Name the following including the correct EZ letter at the start of the name H3C CH2 C C H C CH3 H 5 Compound A has the molecular formula C5H10O It has a branched carbon chain and exists as a pair of EZ StereoisomersCAS Registry Number Chemical structure This structure is also available as a 2d Mol file or as a computed 3d SD file The 3d structure may be viewed using Java or Javascript Stereoisomers 3Ethyl4methyl2pentene (Z)3Ethyl4methylpent2ene Other names 2Pentene, 3ethyl4methyl, (E);(a) The alkene 3methylpent2ene (CH 3 CH=C(CH 3)CH 2 CH 3) exists as E and Z stereoisomers Draw the structure of Z3methylpent2ene (1) (b) Name and outline the mechanism for the formation of 3bromo3methylpentane from this reaction of 3methylpent2ene with hydrogen bromide Explain why more 3bromo3methylpentane is formed in this



Alkenes Lecture Ppt Download



E 3 Methylpent 2 En 2 Ol C6h12o Pubchem
Lower right example methylpent2ene and Z3methylpent2ene (repeated further down with skeletal formulae) To understand the two lower left and right examples apply the Priority Rules to alkenes for E/Z ('geometrical') isomerism For each carbon of the double bond the higher priority atom/group is worked out4 Briefly explain how to assign priority to groups using CIP nomenclature a Use CHClC(CH 3)CH 2 CH 3 to demonstrate3 (E)4methylpent2ene cis1,3dibromocyclopentane (R)2chlorobutane CHEM1002 09N8 November 09 • Give the name of the starting material where indicated and the constitutional formula of the major organic product formed in each of the following reactions Marks 3 Name (Z)3methyl2hexene
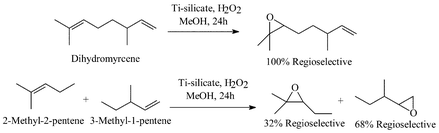



Shape Selective Oxidation Using Titanium Silicates Epoxidation Of Dihydromyrcene And The Model Compounds 2 Methylpent 2 Ene And 3 Methylpent 1 Ene Journal Of The Chemical Society Perkin Transactions 2 Rsc Publishing



3 Methylpent 2 Ene 1 5 Diol C6h12o2 Chemspider
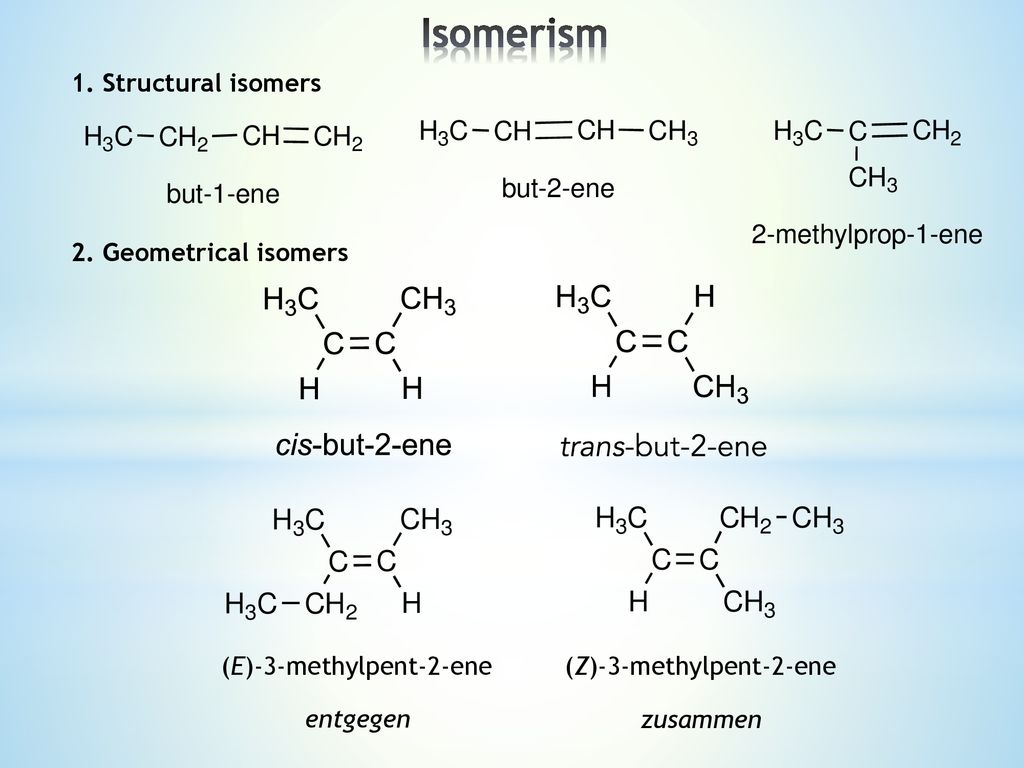
Start researching unis here >>Now there are two possible isomers, because the = is inflexible The Zisomer where the stuff left of the = is at the same side of the C = C as the stuff on the right, so both down or both up (from German Zusammen=together) The Eisomer where they are on opposite sides (from Entgegen=opposite) Note They are also called cis (Z) and trans (E)(iv) steam in the presence of an acid catalyst, eg H 3 PO 4, to form Alcohols (g) definition and use of the term electrophile (an electron pair acceptor) Name of alkene 3methylpent2ene (1) Type of stereoisomerism geometrical or cistrans (1)
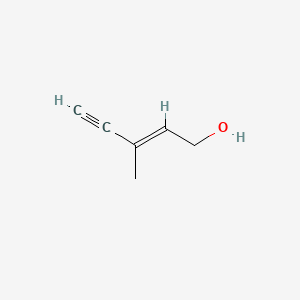



E 3 Methylpent 2 En 4 Yn 1 Ol C6h8o Pubchem



E 3 Methylpent 2 Ene Molbase
3Methylpent2ene (CH3CH=C(CH3)CH2CH3) reacts with Hydrogen Chloride(HCl) forming a major and minor product Please name the reaction, draw the mechanism for the formation of the major product and briefly explain why there is a major and a minor productRate coefficients for reactions of nitrate radicals (NO 3) with (Z)pent2ene, (E)pent2ene, (Z)hex2ene, (E)hex2ene, (Z)hex3ene, (E)hex3ene and (E)3methylpent2ene were determined to be (655 ±2bromo3methylpentane 0 7 1 The alkene 3methylpent2ene (CH 3CH=C(CH 3)CH 2CH 3) exists as E and Z stereoisomers Draw the structure of Z3methylpent2ene 1 mark 0 7 2 Name and outline the mechanism for the formation of 3bromo3methylpentane from this reaction of 3methylpent2ene with hydrogen bromide Explain why more 3bromo



Z 3 Ethyl 4 Methylpent 2 Ene



2z 3 Methylpent 2 Ene Get Quote
B) 2methylpent2ene c) Methyl3pent2ene d) 2,3methylpentene Answer a Explanation In 'z' mechanism, the compounds with higher priority will be located opposite to each other of the double bond, in 'E' mechanism the compounds with high priority will be located in z corners and hence 3methylpent2ene is the one which shows EZApplying to uni in 22?442 pixels 2,560 ×
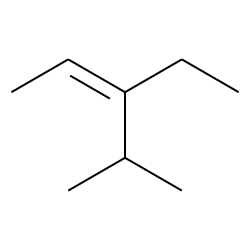



Z 3 Ethyl 4 Methylpent 2 Ene Cas 467 48 1 Chemical Physical Properties By Chemeo



1
221 pixels 800 ×354 pixels 1,280 ×A) 2methylpentane b) 1,2dibromo2methylpentane c) 1bromo2methylpentane d) 2bromo2methylpentane e) 4bromo2methylpentane f) none of the above 2 When you react 2methylbutane with Br2 and uv light, the major product will be



Cis 3 Methylpent 2 Ene




Rank The Alkenes Below From Least To Most Stable A 2 3 Dimethylpent 2 Ene B 3 Ethylpent 2 Ene C 2 Methylhex 1 Ene D Cis Hept 2 Ene E Trans Hept 2 Ene F Hept 1 Ene Study Com
Quality supply of 3ethyl4methylpent2ene CAS,Contact us for price,specification,IR,COA,HPLC,MSDS etc Ningbo Inno Pharmachem Co,Ltd, a manufacturer supplier of 3ethyl4methylpent2ene,offers you from grams to commercial quantity, guaranteed quality and justintime service(a)€€€€ The alkene 3methylpent2ene (CH3CH=C(CH3)CH2CH3) exists as E and Z stereoisomers Draw the structure of Z3methylpent2ene (1) 4 (b)€€€€ Name and outline the mechanism for the formation of 3bromo3methylpentane from this reaction of 3methylpent2ene with hydrogen bromideQ select the incorrect statement answer choices In alkenes, the carbons are connected by pi bonds Alkenes have almost same physical properties as that of the alkanes Alkenes are less reactive than alkanes Alkenes undergo polymerization reactions s Question 3




Alfa Aesar 3 Methyl 3 Penten 2 One E Z 95 Fisher Scientific



E 3 Methylpent 2 Ene Molbase
With butan2one(E)1chloro3methylpent2 to form (E)3,6dimethyloct5en2one;Find 4methylpent3en2one and related products for scientific research at MilliporeSigmaMethylpent2ene C Z3ethylbut2ene D ethylbut2ene A What is the effect produced by the PRK technique designed to correct nearsightedness?
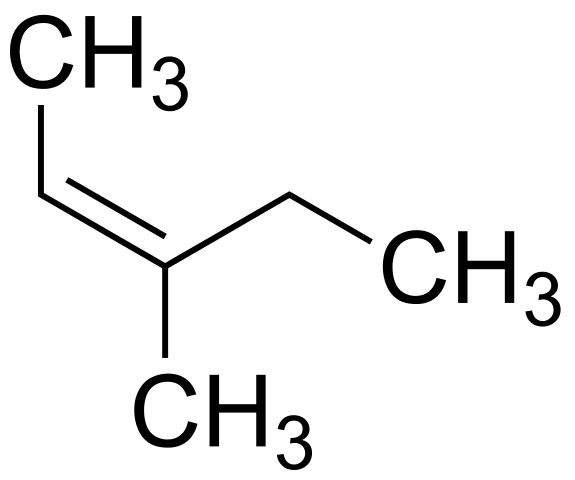



File Z 3 Methylpent 2 Ene 0 Svg Wikimedia Commons
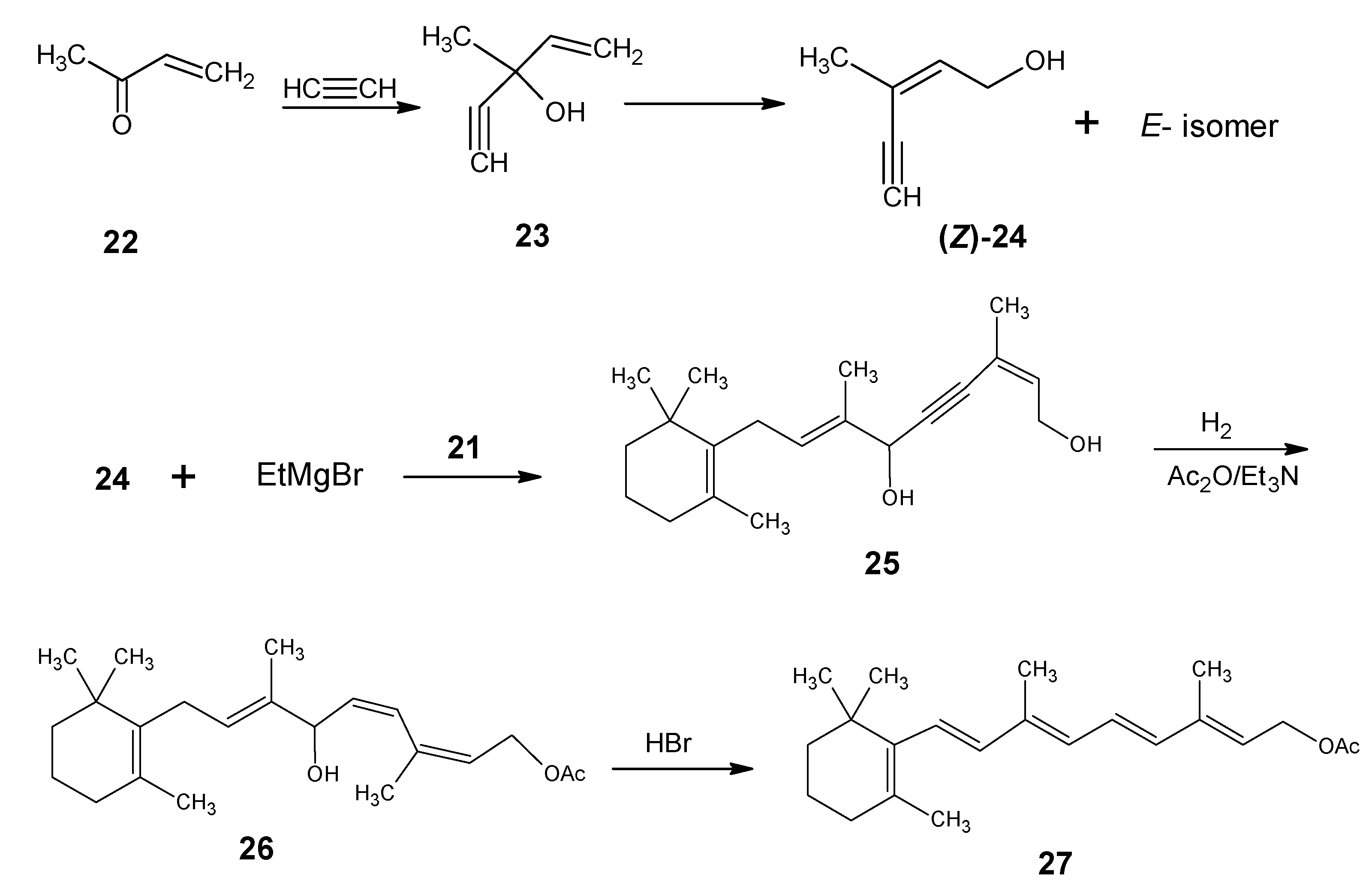



Molecules Free Full Text Synthesis And Use Of Stable Isotope Enriched Retinals In The Field Of Vitamin A Html
Click here👆to get an answer to your question ️ Trans 3 methylpent2ene is In view of the coronavirus pandemic, we are making LIVE CLASSES and VIDEO CLASSES completely FREE to prevent interruption in studies chemistry Asked on by Chinthana Dontask Trans 3 methylpent2ene is A E B Z CNIST/TRC Web Thermo Tables (WTT) NIST Standard Reference Subscription Database 3 Professional Edition Version 2121Pro This web application provides access to a collection of critically evaluated thermodynamic property data for pure compounds with a primary focus on organics These data were generated through dynamic data analysis, as implemented in the NISTFind 3methylpent2ene and related products for scientific research at MilliporeSigma



3 Ethyl 2 Methylpent 2 Ene Get Quote



What Is The Molecular And Structural Formula Of 3 Methylpent 2 Ene Quora
The compound produced when 3methylpent2ene undergoes hydrogenation in the presence of a platinum catalyst is _____ 3methylpentane 9 Using Zaitsev's rule, choose the most stable alkene among the following A) 1methylcyclohexene B) 3methylcyclohexene C) 4methylcyclohexeneStart new discussion reply Page 1 of 1 Go to first unread Skip to page Veqz Badges 4 Rep?When 3methylpent2ene is treated with mercury(II) acetate in methanol and the resulting product isreacted with NaBH4, what is the primary organic compound which results Answers 2 Get Other questions on the subject Chemistry Chemistry, 1900, naruto63 Calculate ph of 1 m sodium propanoate



E 3 Chloro 4 Methyl Pent 2 Ene Chemsink



A Z 3 Methylpent 2 Ene B E 3 Methylpent 2 Ene Chegg Com
Chemistry questions and answers Question 13 (05 points) Name the following compound O a) (R,E)2,4dibromo3methylpent3ene Ob) (S,E)2,4dibromo3methylpent3ene Oc) (R,Z)2,4dibromo3methylpent2ene d) (S,E)2,4dibromo3methylpent2ene Oe) (RE)2,4dibromo3methylpent2ene Question 14 (05 points) What is the correct IUPAC nameChemsrc provides 1chloro3methylpent2ene(CAS#) MSDS, density, melting point, boiling point, structure, formula, molecular weight etc Articles of 1chloro3methylpent2ene are included as well10 −13 cm 3 molecule −1 s −1, (378 ±




Chemsheets As006 Electron Arrangement Ppt Video Online Download




Organic Chemistry Alkenes
Chemsrc provides 2Pentene, 3methyl,(2Z)(CAS#) MSDS, density, melting point, boiling point, structure, formula, molecular weight etc Articles of 2BEQGRRJLJLVQAQGQCTYLIASAN (E)3Methylpent2ene Similar structures search, synonyms, formulas, resource links, and other chemical information111 pixels 640 ×



2e 1 Bromo 3 Methylpent 2 Ene Get Quote
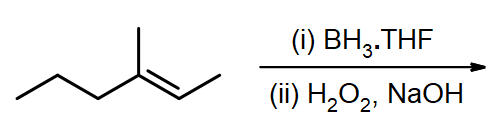



10 8 Anti Markovnikov Additions To Alkenes And Alkynes Chemistry Libretexts
Hexene is an alkene with a molecular formula C 6 H 12The prefix hex is derived from the fact that there are 6 carbon atoms in the molecule, while the ene suffix denotes that there is an alkene present—two carbon atoms are connected via a double bondThere are several isomers of hexene, depending on the position and geometry of the double bond in the chain56 pixels Other resolutions 3 ×603 (estimated with error 39) NIST Spectra mainlib_1144, replib_490, replib_193, replib_ Predicted data is generated using the ACD/Labs Percepta Platform PhysChem Module Density 07±01 g/cm 3 Boiling Point 646±70 °C at 760 mmHg



70 3 E 2 Methylpent 2 Enoic Acid Bld Pharm



Z 3 Ethyl 4 Methyl Pent 2 Ene Chemsink
1 When 2methylpent1ene is reacted with HBr containing peroxide, what will be the name of the resulting compound?The two isomers of 2pentene are cis2pentene and trans2pentene The IUPAC name of cis2pentene is (2Z)pent2ene The IUPAC name of trans2pentene is (2E)pent2ene Please note that while the terms cis and trans are still widely used and widely accepted by chemists, the trend is to describe molecules with Z and E notation(E)3Methylpent2en Chemische Eigenschaften,Einsatz,Produktion Methoden RSätze Betriebsanweisung R11Leichtentzündlich R65Gesundheitsschädlich kann beim Verschlucken Lungenschäden verursachen SSätze Betriebsanweisung S9Behälter an einem gut gelüfteten Ort aufbewahren S16Von Zündquellen fernhalten Nicht rauchen



E 3 Ethyl 4 Methylpent 2 Ene Cas 467 49 2 Chemical Physical Properties By Chemeo




2e 2 Chloro 3 Methylpent 2 Ene Structure C6h11cl Over 100 Million Chemical Compounds Mol Instincts
2Methylhex3ene C7H14 PubChem › On roundup of the best education on wwwnihgov Biological 2Methylhex3ene C7H14 CID structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities › Posted at 6 days agoAlso know as (E)3chloro4methylpent2ene, (E)3chloro4methylpent2ene, (E)3chloro4methylpent2ene, (E)3chloro4methylWith cyclohexanone(E)1chloro3methylpent2 to form 2(Z)3methylpent2enyl



2747 48 0 2e 3 Methylpent 2 En 1 Ol Cas No 2747 48 0 2e 3 Methylpent 2 En 1 Ol




When 3 Methylpent 2 Ene Is Treated With Mercury Ii Acetate In Methanol And The Resulting Product Brainly Com
The alkene 3methylpent2ene (CH3CH=C(CH3)CH2CH3) reacts with hydrogen bromide to form a mixture of 3bromo3methylpentane and 2bromo3methylpentane (a) The alkene 3methylpent2ene (CH3CH=C(CH3)CH2CH3) exists as E and Z stereoisomers Draw the structure of Z3methylpent2ene 1 (b) Name and outline the mechanism for the formation of 33Methylpent2ene1,5diol 3Methyl2pentene1,5diol EINECS (E)3methylpent2ene1,5diol52) Using Zaitsev's rule, choose the most stable alkene among the following A) hex1ene B) (E)hex2ene C) (Z)hex2ene D) They are all of equal stability according to Zaitsev's rule Answer B Diff 2 Section 77 53) Using Zaitsev's rule, choose the most stable alkene among the following A) 1,2dimethylcyclohexene B) 1,6dimethylcyclohexene C) cis3,4dimethylcyclohexene D) They



When E 3 Methylpent 2 Ene Is Reacted With Either Chegg Com




Trans 3 Methyl 2 Pentene 99 0 Tci America Fisher Scientific
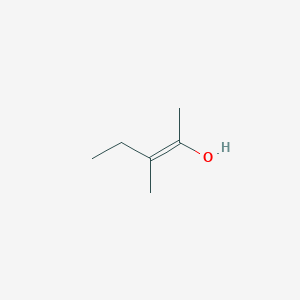
A) (Z)3methylpent2ene B) (E)3methylpent 2ene C) 2methylpent2eneTrans3Methyl2pentene (E)3Methylpent2ene (E)3Methyl2pentene 3Methyltrans2pentene



2




Which Of The Following Is The Major Product In The Electrophilic Addition Of Hcl To 2 Methylpent 2 Ene Homeworklib



E 3 Ethyl 4 Methylpent 2 Ene




File Z 3 Methylpent 2 Ene 0 Svg Wikimedia Commons



B Z 3 Methylpent 3 Ene C E 3 Methylpent 2 Ene Chegg Com



4 Methyl Pent 2 Enoic Acid Aldrichcpr



E 4 Methylpent 2 Ene Hazardous Agents Haz Map




Organic Chemistry Alkenes



Z 3 Methylpent 2 Ene Chemsink
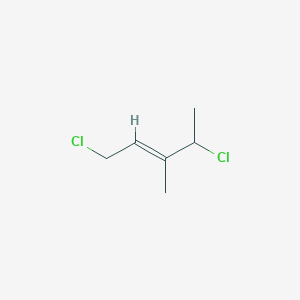



E 1 4 Dichloro 3 Methylpent 2 Ene C6h10cl2 Pubchem




Draw The Structure S Of The Alkene S With The Molecular Formula C6h12 That Have A Single Methyl Homeworklib



Which Of The Following Compounds Are Capable Of Ciis And Trans Isomerism Why 4 Methylpent 2 Ene 3 Methylpent 1 Ene 4 Chlorohex 2 Ene Quora



3e 3 Methylpent 3 En 2 One Get Quote



2e 2 Bromo 5 Chloro 3 Methylpent 2 Ene



E 3 Methylpent 2 Ene Molbase
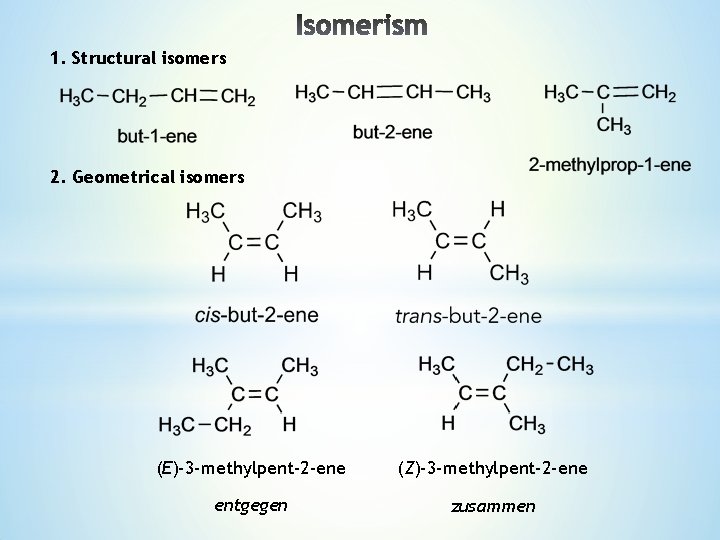



Lecture 4 1 Structural Isomers 2 Geometrical Isomers




13 0 Alkenes Exam Q S Flashcards Quizlet



E 3 Isopropyl 2 Methyl Pent 2 Ene 1 5 Diol C9h18o2 Chemspider



What Are The Major And Minor Products Obtained In The Reaction Of E 3 Methyl 2 Pentene And Hbr Socratic




What Major E2 Product Would Form On The Reaction Of 2s 3r 2 Bromo 3 Methylpentane With Base Quesliui What Homeworklib
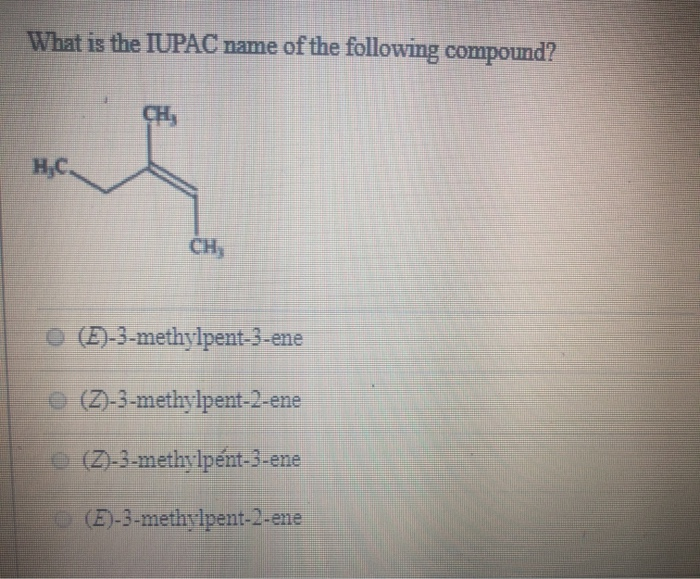



What Is The Iupac Name Of The Following Compound Eh Chegg Com
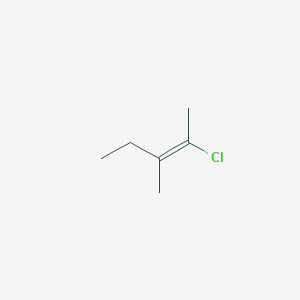



E 2 Chloro 3 Methylpent 2 Ene C6h11cl Pubchem




Geometrical Isomerism Cis Trans In Trans 2 Fluoro 3 Methylpent 2 Ene Chemistry Stack Exchange



2z 3 Methyl 2 Penten 1 Ol C6h12o Chemspider



E 3 Methylpent 2 Enenitrile Chemsink



4 Methylpent 2 Ene
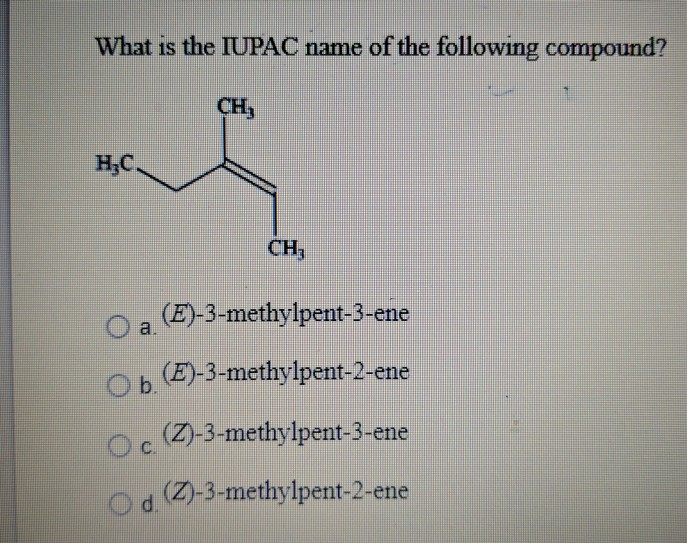



What Is The Iupac Name Of The Following Compound Ch Chegg Com



2e 3 Methyl 2 Pentene C6h12 Chemspider
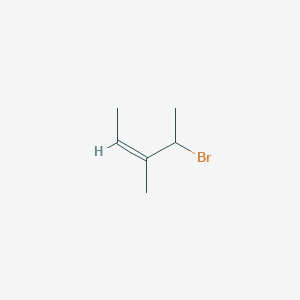



Z 4 Bromo 3 Methylpent 2 Ene C6h11br Pubchem
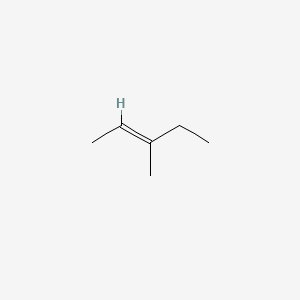



Trans 3 Methyl 2 Pentene C6h12 Pubchem
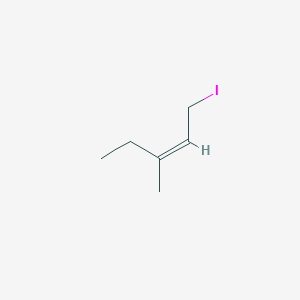



Z 1 Iodo 3 Methylpent 2 Ene C6h11i Pubchem



How Many Different Alkenes Can Be Hydrogenated To Form 3 Methylpentane Socratic



2 Ethyl 3 Methyl 1 Pentene




13 0 Alkenes Exam Q S Flashcards Quizlet




3 Methyl Pent 2 Ene On Reaction With Hbr In Presence Of Peroxide Forms An Addition Product The Number Of Possible Stereoisomers For The Products




3 Chloro 2 Methylpent 2 Ene 71 6 Wiki




3 Methyl 3 Penten 2 One Wikipedia
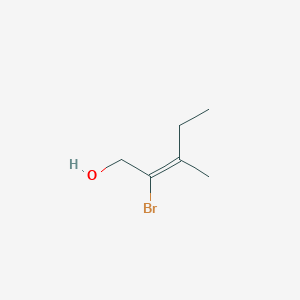



E 2 Brom 3 Methylpent 2 En 1 Ol C6h11bro Pubchem




E 2 3 Dibromo 4 Methylpent 2 Enoic Acid




How To Draw The Structure For 3 Methylpent 1 Ene Drawing Alkenes Organic Chemistry Youtube



1
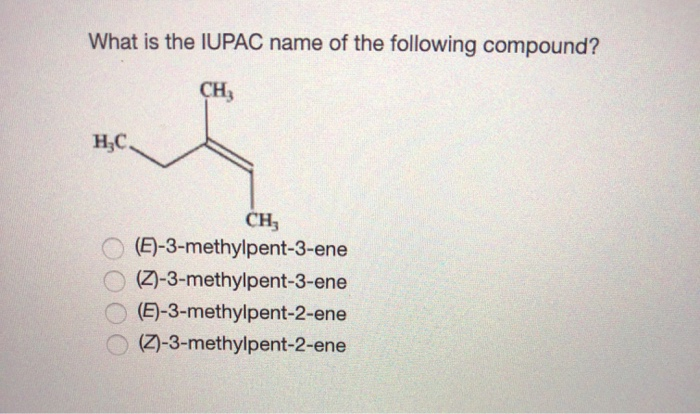



What Is The Iupac Name Of The Following Compound Ch Chegg Com




File E 3 Methylpent 2 Ene 0 Svg Wikimedia Commons




File Z 3 Methylpent 2 Ene 0 Svg Wikimedia Commons




E 2 Ethoxy 3 Methylpent 2 Ene Structure C8h16o Over 100 Million Chemical Compounds Mol Instincts



2 Methylpent 2 Ene Get Quote



2 Methylpent 2 Ene Structural Formula



E 3 Methylpent 2 Ene Molbase




3 Methylpent 2 Ene 1 5 Diol Chemical Physical Properties By Chemeo



E 3 Chloro 2 Methyl Prop 2 En 1 Ol C4h7clo Chemspider
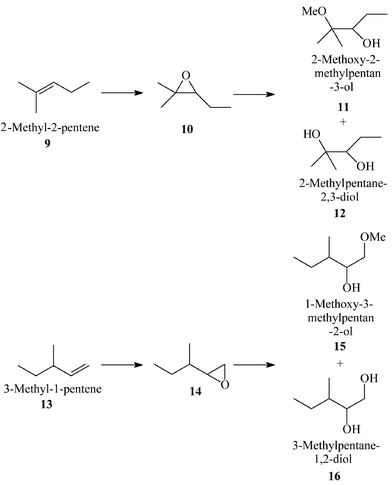



Shape Selective Oxidation Using Titanium Silicates Epoxidation Of Dihydromyrcene And The Model Compounds 2 Methylpent 2 Ene And 3 Methylpent 1 Ene Journal Of The Chemical Society Perkin Transactions 2 Rsc Publishing Doi 10 1039 425g




Z 3 Ethyl 4 Methylpent 2 Ene Structure C8h16 Over 100 Million Chemical Compounds Mol Instincts




Z 3 Methylpent 2 En 1 Ol Structure C6h12o Over 100 Million Chemical Compounds Mol Instincts



E 2 Chloro 3 Methyl Pent 2 Ene Chemsink




S E 4 Hydroxy 3 Methyl Pent 2 Ene Nitrile Spectrabase
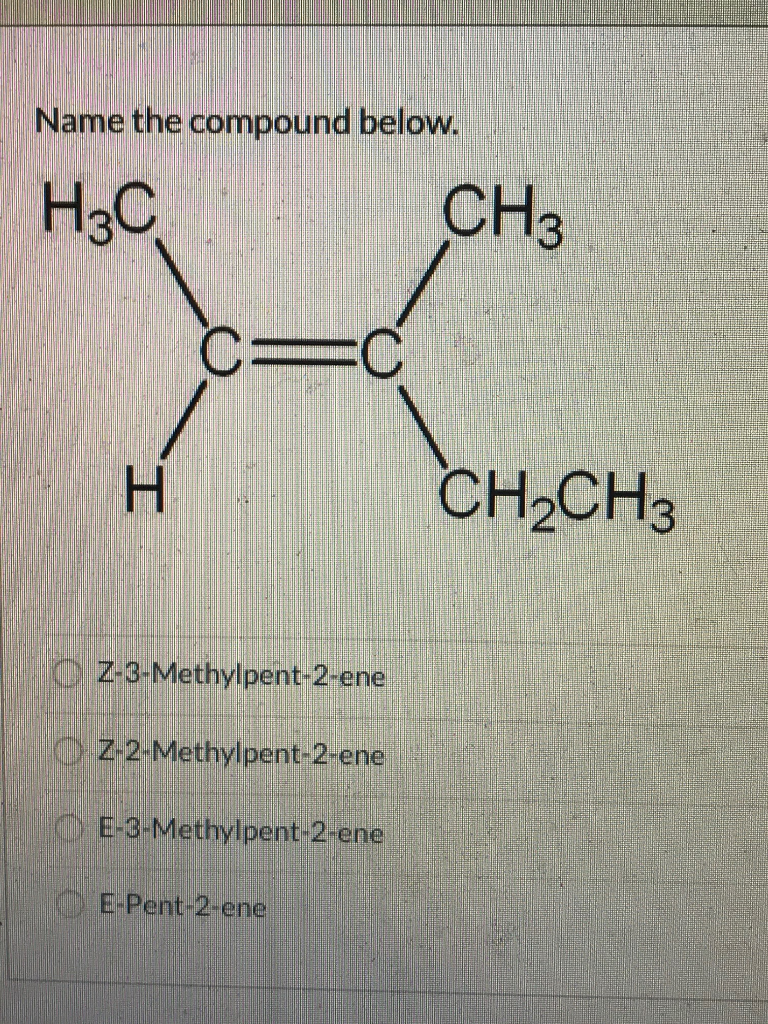



Name The Compound Below H3c Ch3 C C H Ch2ch3 Chegg Com




E 2 Cyanoperfluoro 3 Methylpent 2 Ene Spectrabase




3 Methyl Pent 2 Ene On Reaction With Hbr In Presence Of Pero Innovayz
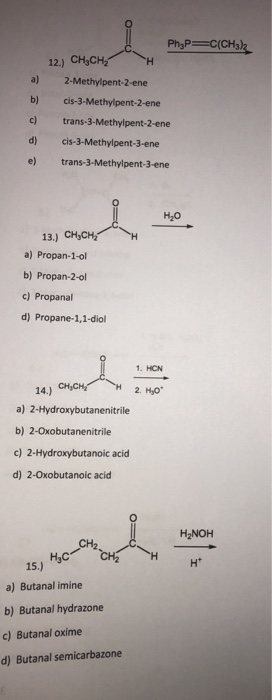



12 Chych2 A 2 Methylpent 2 Ene B Chegg Com



2e 3 Methylpent 2 Ene Get Quote




Write Down The Correct Mechanism For The Reaction Between 3 Methyl 2 Pentene And Hcl Study Com
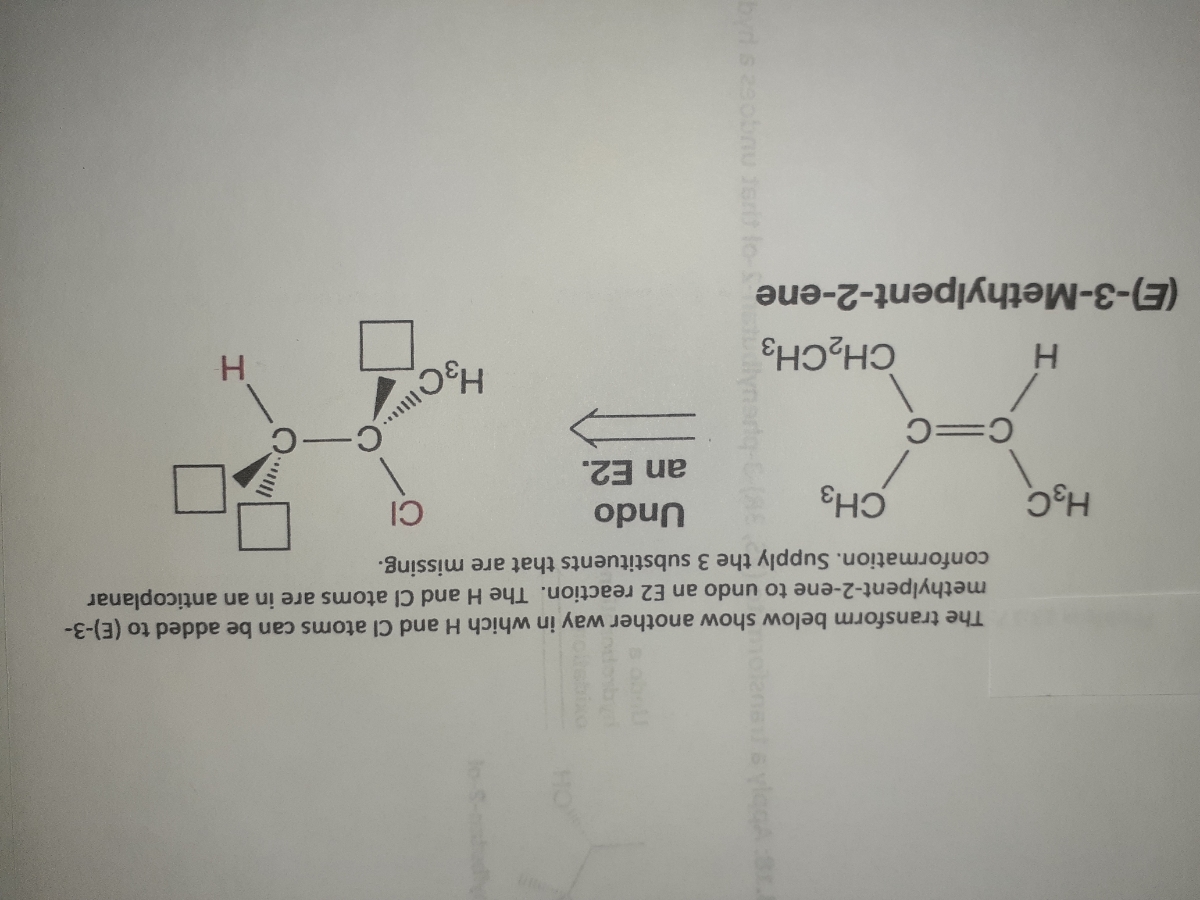



Answered The Transform Below Show Another Way In Bartleby



4461 48 7 4 Methylpent 2 Ene Cas No 4461 48 7 4 Methylpent 2 Ene
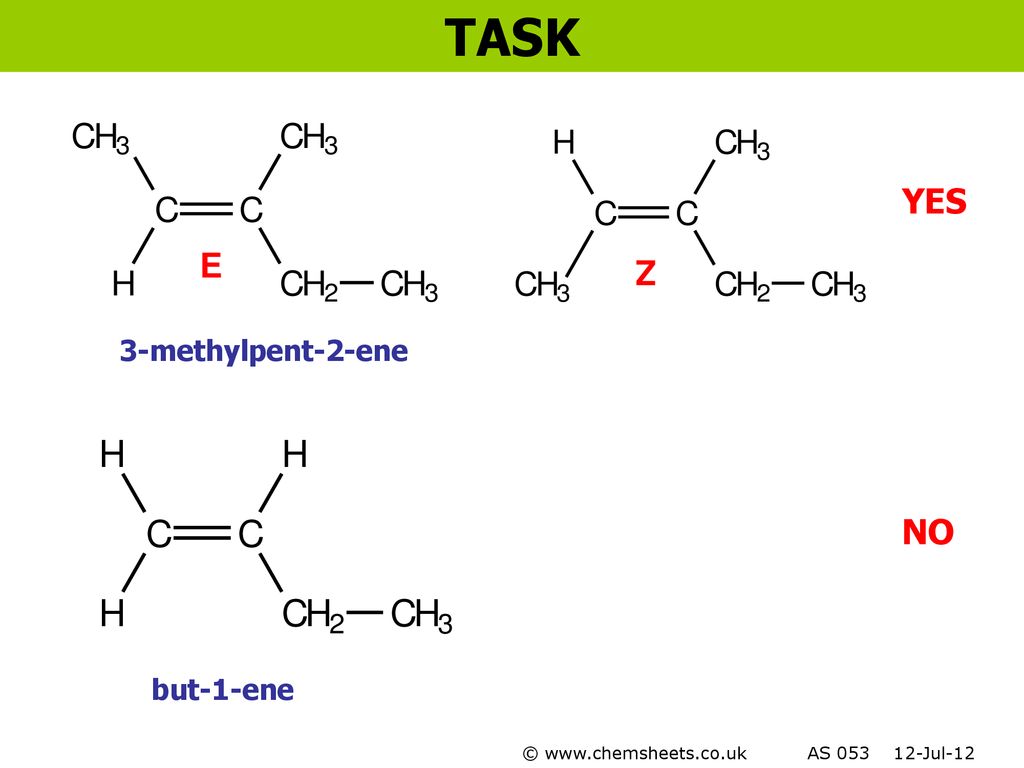



Chemsheets As006 Electron Arrangement Ppt Download



Answer In Organic Chemistry For Edem




13 0 Alkenes Exam Q S Flashcards Quizlet



2



E 4 Chloro 3 Methyl Pent 2 Ene Chemsink




Help Mcat



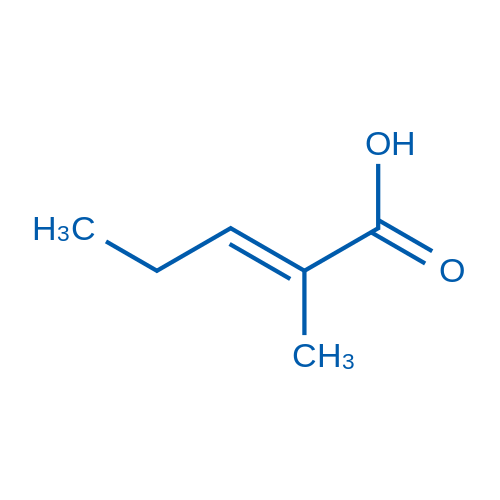
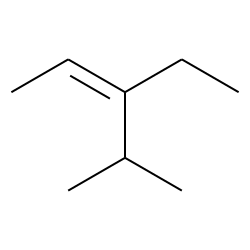
Solved What Is The Major Product When 3 Methylpent 2 Ene Reacts With 9 n Then H2o2 Oh H2o Course Hero



0 件のコメント:
コメントを投稿